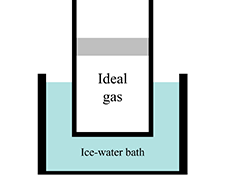
Ideal gas law
Variant i Interactive tutorial lecture Other Variants Dynamics first
Students apply the concepts of pressure, temperature, and volume to an ideal gas and determine how these change when forces are exerted on a gas.
Topics Thermal and statistical / Thermodynamics: graphs, proportional reasoning, representations, atmospheric pressure, forces, free-body diagrams, Ideal gas law, Newton's second and third laws, pressure, pV diagrams, temperature, and volume
Materials
Materials by the UW team
- Clicker Questions Only

- Instructor Guide

- Pretest


- Pretest for LMS


- Exam Questions


- Equipment List

Tutorial details
Section I: Pressure
In this section, students consider a gas sample in a cylinder sealed with a piston of mass $m$ that can move vertically without friction. They draw free-body diagrams for the piston and apply Newton’s second law to relate the forces acting on the gas. By relating these forces to pressures, they determine that the pressure of the gas must be greater than atmospheric pressure. In Question G, they recognize that the pressure of the gas depends only on the characteristics of the piston (mass, cross-sectional area) and the atmospheric pressure.
Section II: Ideal gas law
Students describe the temperature, volume, & pressure of different gas samples. They apply the results from Section I along with the ideal gas law to aid in their reasoning. In Question F, they consider a student dialogue that includes two incorrect applications of the ideal-gas law.
Section III: $\textbf{pV}$ diagrams
In this section, students draw a $pV$ diagram for a process seen in Section II. They compare the pressures, volumes, and temperatures of different states of a gas using $pV$ diagrams.
For instruction tips, login or register as a verified educator to see the Instructor Guide.
Prerequisites
Prerequisite tutorials
The Newton's second and third laws tutorial is a prerequisite to Ideal gas law.
Other prerequisites
It is assumed that the relationship between force and pressure has been introduced. The tutorial requires students to draw free-body diagrams and apply Newton’s second law to an object at rest.
Research
- C. Kautz, P. Heron, M. Loverude, and L. McDermott, Student understanding of the ideal gas law, Part I: A macroscopic perspective, Am. J. Phys. 73 (11), 1055 (2005).
- C. Kautz, P. Heron, P. Shaffer, and L. McDermott, Student understanding of the ideal gas law, Part II: A microscopic perspective, Am. J. Phys. 73 (11), 1064 (2005).
Coming Soon! We hope to release the discussion section on each tutorial soon.

