First law of thermodynamics
Variant i Interactive tutorial lecture Other Variants Dynamics first
Students are led to distinguish between heat transfer and work as means of changing the thermal energy of a gas. Failure to distinguish related concepts is also addressed.
Topics Thermal and statistical / Thermodynamics: graphs, First law of thermodynamics, Ideal gas law, pressure, pV diagrams, thermal energy, volume, and work
Materials
Materials by the UW team
- Clicker Questions Only

- Instructor Guide

- Pretest


- Pretest for LMS


- Exam Questions


- Equipment List

Tutorial details
Section I: Work
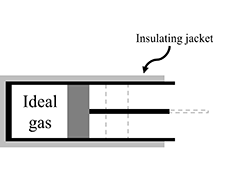
The tutorial begins with a brief review of mechanical work. In Questions A-E, students are guided to think about the sign of the work done by a movable piston on a gas contained in a cylinder. They consider whether the answer depends on the motion of the piston.
Section II: Work & thermal energy
Students are presented with the relationship between the work done on a thermally insulated ideal gas system and the change in thermal energy of that system. In Question A they apply that relationship to an adiabatic compression.
Question B involves the isochoric heating of an ideal gas. Students consider how different quantities change during the process, then sketch a $pV$ diagram.
Section III: Heat, work, and thermal energy
Students now consider the first law of thermodynamics and recognize that the previous processes were special cases where one term was zero. They then apply the first law to an isothermal process.
For instruction tips, login or register as a verified educator to see the Instructor Guide.
Prerequisites
Prerequisite tutorials
The Ideal gas law tutorial is a prerequisite to First law of thermodynamics.
Other prerequisites
The tutorial assumes that the students have been introduced to the ideal gas law and the first law of thermodynamics.
Research
- M. Loverude, C. Kautz, and P. Heron, Student understanding of the first law of thermodynamics: Relating work to the adiabatic compression of an ideal gas, Am. J. Phys. 70 (2), 137 (2002).
Coming Soon! We hope to release the discussion section on each tutorial soon.

